
Лип . 18, 2024 14:33 Back to list
THE BENEFITS OF HOT DIP GALVANISED STEEL FENCING
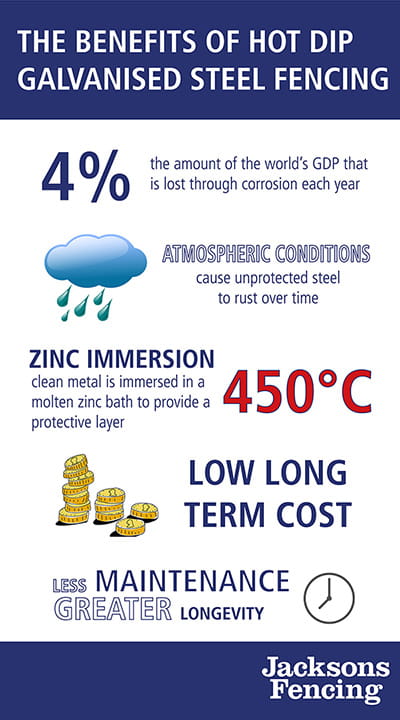
It’s estimated that up to 4% of the world’s GDP is lost through corrosion each year. When installing a steel fence, rust prevention should always be a key consideration; the materials and methods used will have a major impact on the longevity of your fence.
One of the best ways of protecting against corrosion is hot-dip galvanisation: a process in which the entire fence surface is dipped into molten zinc, coating it completely. This provides a barrier protection between the metal and its surrounding environment.
THE SCIENCE OF GALVANISATION
Without protection, steel will rust over time due to atmospheric conditions, with the degree of corrosion depending on the environment that the product is in. Steel is made mainly from iron and the rust is an iron oxide (typically a red oxide) which is formed by the reduction and oxidation reaction of iron and oxygen, in the presence of moisture.
Galvanisation is highly effective in preserving your fence because the corrosion of zinc, which protects the base metal, is very slow. A deep scratch in the surface of the zinc will expose the underlying metal; a galvanic cell is formed at this location, around which the zinc corrodes but still protects the metal – a process known as cathodic protection. The zinc acts as an anode and provides free electrons, sacrificing its ions and keeping the less active metal underneath from rusting. A zinc coating therefore provides ‘sacrificial protection’.
Other protective methods include painting or plastic coating, but these have serious flaws if used as the only method of protection and being applied directly to the steel substrate. When damaged, these inferior coatings can fall away, making them unreliable and in need of constant maintenance.
THE HOT-DIP GALVANISING PROCESS
The galvanising process has a number of stages, all by immersion, that are required to achieve the final finish:
DEGREASING
Acid or alkaline-based proprietary products may be used and they may be heated or used at ambient temperatures.
Powder Coated Palisade Fence
PICKLING
This is done in dilute hydrochloric acid which dissolves rust and produces a chemically clean surface.
FLUXING
A mixture of zinc chloride and ammonium chloride in solution is the standard agent of choice. The steelwork must be dry on completion of this stage.
ZINC IMMERSION
The final stage involves the clean metal being immersed in a special bath of molten zinc at 450°C where the zinc alloys with the iron in the steel to form zinc/iron alloy layers. These layers are the basis of the coating.
polyester powder coating can be applied if required after galvanising to protect the steel from rusting which allows the steel to not peel off with the paint still stuck to it. For installations near coastal areas, a marine coating can ensure that moisture does not get through to the galvanising below, helping to protect the finished product.
THE BENEFITS OF HOT-DIP GALVANISATION
Being a well-established process that’s helped combat steel corrosion for over a century, the benefits of galvanisation have been proven extensively. The main benefits are as follows:LOW LONG-TERM COST
Even in cases where the initial cost of galvanising is higher than alternative coatings, galvanising is always cheapest in the long term because it lasts longer and doesn’t require constant care.
LESS MAINTENANCE
Galvanising offers complete coverage, sealing the base metal and stopping corrosion for good, eliminating the need for continual maintenance. The long-term durability is achieved at relatively low environmental burden.
GREATER LONGEVITY
Galvanising provides maximum protection through a tough, abrasion-resistant coating of increased thickness.
The lifespan of a zinc coating is largely determined by its thickness.
-
Powder Coated Double Wire Mesh Fence - Anping County Shengxin Metal Products Co., Ltd
NewsAug.03,2025
-
Power Coated 358 Anti Climb Mesh Fence for Airports
NewsAug.03,2025
-
Powder Coated Double Wire Mesh Fence-Anping County Shengxin Metal Products Co., Ltd.
NewsAug.02,2025
-
Powder Coated Double Wire Mesh Fence | Anping County Shengxin Metal Products Co., Ltd
NewsAug.02,2025
-
Powder Coated Double Wire Mesh Fence for Germany Market-Anping County Shengxin Metal Products Co., Ltd|Durability, Aesthetics, Compliance
NewsAug.02,2025
-
Powder Coated Double Wire Mesh Fence-Anping County Shengxin Metal Products Co., Ltd.|Durability&Compliance
NewsAug.02,2025

